The number of refreshing medicines is increasing. These products demand respect for the cold chain, particularly to limit temperature excursions. Thermis Log 1 enables you to measure and record precise temperature data in the blink of an eye.

According to the Cercle de réflexion de l'industrie pharmaceutique (Crip), one out of every two drugs on the market is heat sensitive. Behind this boom in heat-sensitive health products (TSPs) lies a whole range of logistics linked to compliance with the cold chain required for their movement and storage, with both financial and human issues in case of temperature variations. These can become not only inefficient, but dangerous.
Indeed, they can be affected by the degradation of their active ingredient, by the modification of their excipients, by the deterioration of their packaging or their packing. In any case, because of abuse or lack of treatment, they put the patient at risk. Temperature impacts on health products can be microbiological, chemical, physical or physicochemical. A distinction must be made between the effects of high temperatures, the "effects of heat", and the effects of low temperatures, the "effects of cold".
Growth hormones, insulins, anti-cancer drugs, many vaccines, ... all these drugs are heat sensitive and must be stored in controlled temperatures to be effective. Exposing them to temperatures below +2°C or above +8°C is harmful to the product and may be harmful to the patient. Especially since these medicines are high-value treatments that affect the health of the patient and the economics of the manufacturer, pharmacy or hospital that delivers them. The share of heat-sensitive drugs in the pharmaceutical market is growing steadily and is expected to evolve for the main reason: Increased investment in R&D on innovative molecules from biotechnologies for the development of specialized treatments against cancer or diabetes, which meet a growing demand worldwide.

Today, the logistics challenges of the temperature-controlled healthcare distribution chain are becoming more diverse and complex. The amounts stored and transported also range from one dose to millions of doses. The internationalization of the market, the globalization of exchanges, the specifications of certain producers, the development of generics and numerous new molecules, in particular in biotechnologies, also lead to an evolution of the logistics of the laboratories, as well as a strong increase in the needs for controlled temperature over durations sometimes exceeding several weeks.
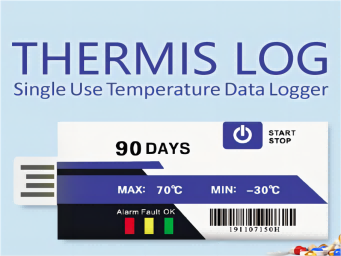
As a result, the data logger is essential for the transportation and storage of health products. Thermis Log 1 is a single-use temperature recorder specially designed for the pharmaceutical industry. With an accuracy of 0.3℃, this logger is ideal for recording temperature-sensitive shipments, including vaccine shipments. The sensor is cheaper, easy to operate, space-saving and generates non-modifiable PDF data reports by plugging the recorder into a PC. We offer a variety of models with different capabilities (7, 15, 30, 60 or 90 available) to meet the specific needs of each application. Compliance with the whole cold chain, from production to delivery, is essential to ensure the quality of the product, as well as to avoid financial losses or reputational damage.

 English
English Español
Español Русский
Русский Français
Français Deutsch
Deutsch عربي
عربي 中文
中文



